Navinci is a strong research organization and as the center of excellence for proximity ligation technology, we continue to innovate the technology with new developments and improvements. A number of additional patent applications have been added, and we now have a broad product portfolio based on our Naveni Proximity Ligation Technology. See all products here.
Examples of the latest innovations are several. Naveni™TriFlex, a revolutionary proximity-based technology that concurrently visualizes two proteins in a free and interacting within any cell compartment. The detected protein A, B and the interaction of A/B signals are amplified and generate fluorescent readout in three different channels corresponding to each protein pool. The TriFlex method gives more information from the same experiments and will make it possible to unravel e.x. protein degradation and protein interplay. Learn more about Naveni™TriFlex here.
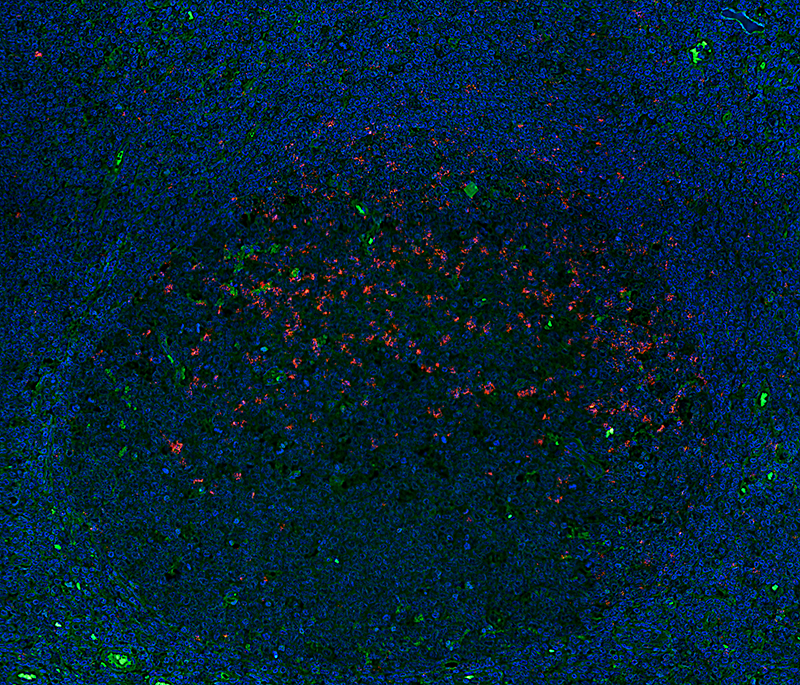
With a proprietary solution, we have also solved a difficult problem in the immunofluorescent staining of tissues. Tissues are complex multicellular structures where fluorescently labeled detection reagents have been observed to bind unspecifically to certain cell structures resulting in background staining that can be difficult to distinguish from true biologically relevant staining. To address this problem, we developed a new proprietary version of NaveniFlex, highly optimized for tissue use. The product line tailor-made for tissue is called NaveniFlex Tissue.
We are experts in assay development and as we know that it takes time to find the optimal primary antibodies. We have expanded our product line of target-specific kits with primary antibodies included. Our latest focus is on target-specific kits within immunooncology, we have a kit for the detection of PD1/PD-L1 interaction, designed to help researchers better understand the interactions between immune checkpoint proteins and cancer cells and how these interactions play a role in the development and treatment of cancer. Read more about PD1 signaling.
Phosphorylation of proteins are important part of the signaling cascades, but studying phosphorylations has been difficult due to the unspecificity of phospho antibodies. The target-specific Naveni PTMs utilized the specificity with Proximity Ligation Assay and includes validated primary antibodies to detect phosphorylated proteins such as PD1, VEGFR2, HER2 etc. To Target specific kits.
In conclusion, the proximity ligation assay has become an indispensable tool in biomedical research, providing researchers with a fast, efficient, and sensitive method to study protein interactions and protein localization on single-cell level. With innovations like the Naveni technology leading the way in the development and commercialization of proximity ligation assay technology, the future of biomedical research looks bright.