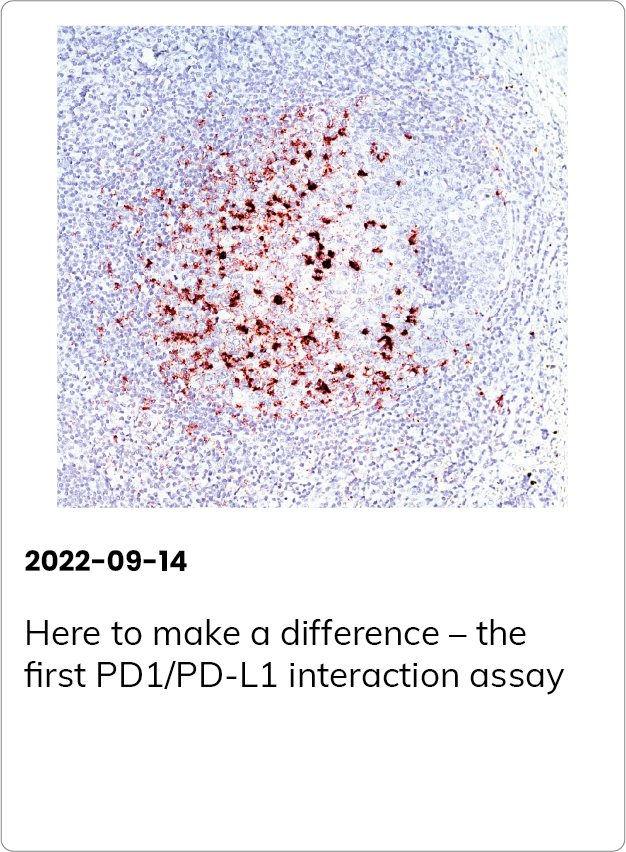
Here to make a difference – the first PD1/PD-L1 interaction assay
Immune checkpoint inhibitors have revolutionized cancer immunotherapy, and more than 3000 anti-PD1/PD-L1 therapies are currently in clinical trials. The leading technology used to determine whether a patient is likely to benefit from PD1/PD-L1 immunotherapy is PD-L1 immunohistochemistry. However, this test doesn’t reveal whether the interaction between PD1 and PD-L1 actually occurs, and correlates poorly with patient response to treatment.
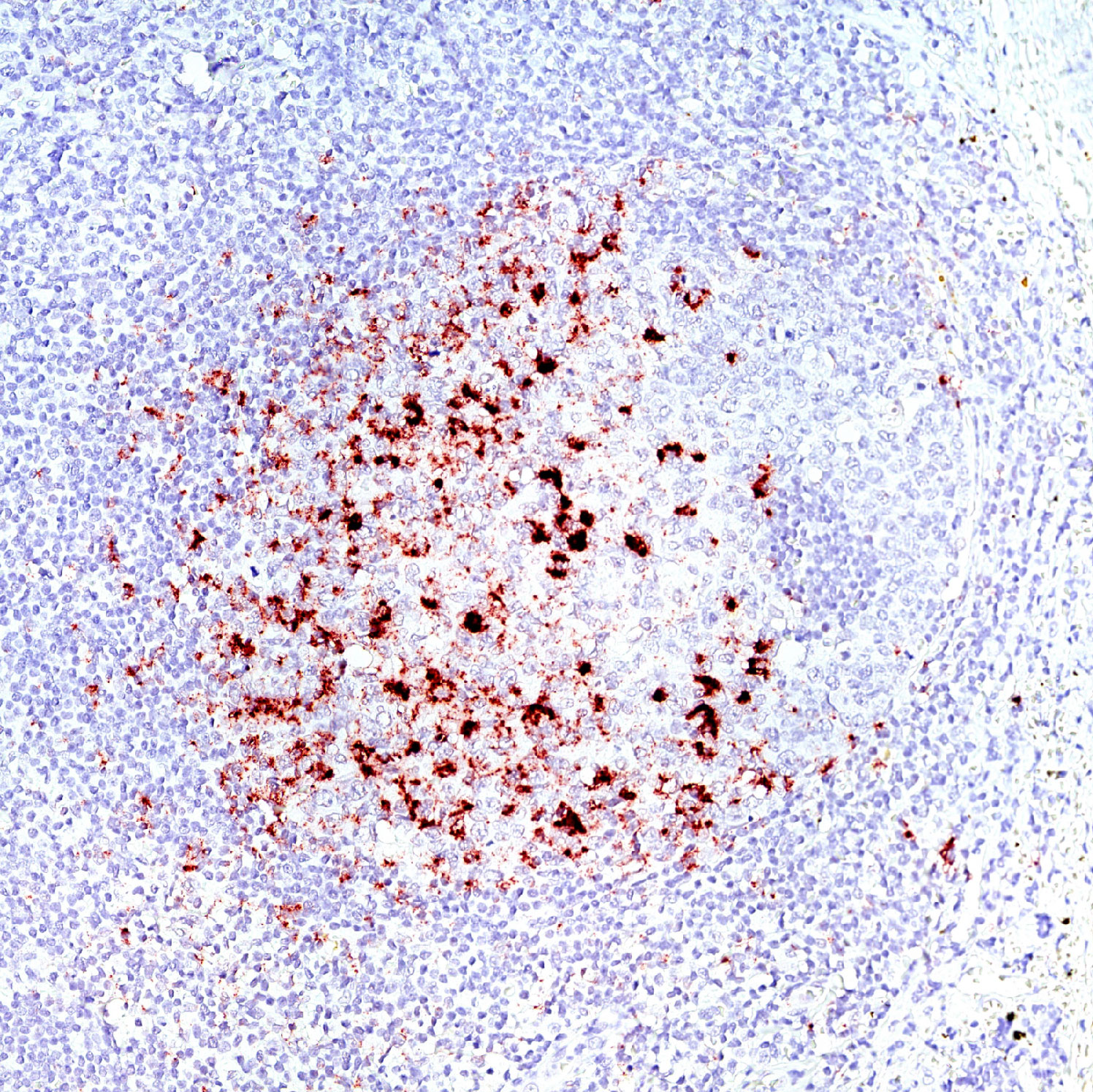
PD1/PD-L1 interaction has predictive value for patient prognosis and survival, but up until now, the possibilities to detect it in tissue samples have been very limited.
Here is how our newly developed in situ assay makes a difference:
Naveni PD1/PD-L1 is a Proximity Ligation Assay for the specific detection of the PD1/PD-L1 interaction in situ. The assay can be performed on FFPE and fresh frozen tissue samples. The assay has been verified in several different human FFPE tissues, such as Hodgkin’s lymphoma, Tonsil, Lung acinar adenocarcinoma, Lung squamous cell carcinoma, Malignant Melanoma, Colon adenocarcinoma, and Pancreatic ductal carcinoma.